Dietary Supplementation of Dunaliella Salina on Growth Performance and Body Composition of Indian White Shrimp Fenneropenaeus Indicus (H Milne Edwards)
Osamah A Ahmad, Adnan J Salama, Sambhu Chithambaran*
Affiliation
Department of Marine Biology, Faculty of Marine Science, King Abdulaziz University, Jeddah, Saudi Arabia
Corresponding Author
Chithambaran, S. Department of Marine Biology, Faculty of Marine Science, King Abdulaziz University, Jeddah, Saudi Arabia. Tel: +966552790434; E-mail: sambhu@kau.edu.sa
Citation
Chithambaran, S., et al. Dietary supplementation of Dunaliella salina on growth performance and body composition of Indian white shrimp, Fenneropenaeus indicus (H. Milne Edwards). (2015) J Marine Biol Aquacult 1(1): 1- 5.
Copy rights
© 2015 Chithambaran, S. This is an Open access article distributed under the terms of Creative Commons Attribution 4.0 International License
Keywords
Dunaliella Salina;Fenneropenaeus Indicus;White Shrimp;Dietary
Abstract
Juveniles of white shrimp, Fenneropenaeus indicus were fed with a micro green alga, Dunaliella salina through a fishmeal based supplementary diet containing 35% protein for a period of 180 days in fiberglass tanks. There were control and treatments for the study and both were triplicated. D. salina was incorporated at 0.5, 1.0, 2.0, 4.0 and 8.0% in the diet and was designated as treatments T1, T2, T3, T4 and T5 respectively. D. salina free diet was considered as Control. The diet containing D. salina at 2.0% (T3) showed significantly (p < 0.05) superior growth when compared to control and other treatments. Protein efficiency ratio and protein digestibility were found to be high in T3 and feed conversion efficiency was reduced. Better colour and taste were observed in D. salina fed shrimp compared to control. Crude protein, lipid, ash and nitrogen free extract (NFE) of the carcass did not show significant difference between control and treatments. Dietary administration of D. salina enhances growth and body colour in white shrimp F. indicus.
Introduction
The production of natural feed additives for the aquaculture industry is a thriving sector. These substances are being used for several purposes including the enhancement of the immune systems of farmed shrimp, promoting growth, attaining the desired flesh and skin pigmentation, as well as improving the organoleptic properties of the farmed product[1,2]. Astaxanthin is the major carotenoid responsible for the pink-red pigmentation of many fish and shrimp species[3]. Carotenoids are a source of pro-vitamin A which increase survival rate, weight gain and disease resistance against white spot syndrome virus in shrimp[4,5]. Carotenoids not only control ammonia level and resistance to stress but also improve hepato-pancreatic function, cholesterol and polyunsaturated fatty acids from oxidation and provide antioxidant protection[6-8]. Among the carotenoids, astaxanthin was found to be more effective than β-carotene as an antioxidant and a better agent to destroy free radicals than other carotenoids[9,10].
Dunaliella salina is a microalgae occurring naturally in a number of locations worldwide. In the marine environment, D. salina appears green, however, in conditions of high salinity and light intensity, the microalgae turns red due to the production of protective carotenoids in the cells[11]. The majority of harvested micro-algae are currently being sourced from one of the most pristine environments in the world – a remote coastal salt lagoon in Western Australia[12]. D. salina is harvested without any harmful solvents or chemicals and the carotenoids (highly-prized anti-oxidant pigments responsible for the red color) are then extracted for use in pharmaceuticals, cosmetics, nutritional supplements, aquaculture feeds and food coloring[13]. D.salina paste has a wide range of applications as feed stock. As a direct feed for filter feeders such as corals and sponges, as an enrichment for live feed organisms such as Artemia and Rotifers, especially for marine ornamental fish where the coloration is an important consideration, as an enrichment in fresh food (Mussels, Pipis, etc.) for crustacean larvae and brood stock, as a natural source of carotenoids in diets for shrimps and fish[14,15]. Considering the importance of D. salina as a feed additive in aquaculture, the present study was conducted to evaluate the effect of D. salina on growth, pigmentation and body composition in Indian white shrimp, Fenneropenaeus indicus an ideal candidate species for coastal aquaculture practice in the Kingdom.
Results
Growth Performance
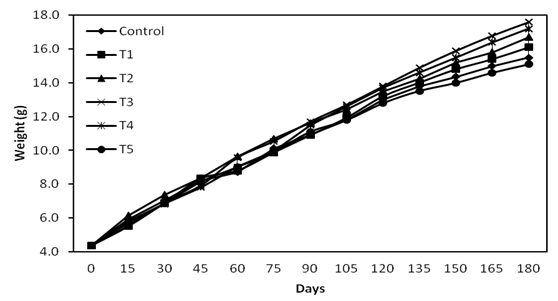
Data on growth performance of shrimp are presented in Table 1 and Figure 1. A diet containing D. salina at 2.0% (T3) showed superior growth when compared to control and other treatments. ANOVA shows that there is significant difference (p < 0.05) on weight between control and treatments. High SGR (%) was observed in T3 followed by T4, T2 and T1 and it was low in T5. Biomass was high in T3 compared to control and other treatments. Highest survival was recorded in Control followed by T1, T3, and T4 and it was lowest in T2.
Table 1: Growth, survival and biomass of shrimp fed D. salina diets
| Parameters | Control M ± SD | T1 M ± SD | T2 M ± SD | T3 M ± SD | T4 M ± SD | T5 M ± SD |
|---|---|---|---|---|---|---|
| Initial length (cm) | 9.2 ± 0.1 | 9.2 ± 0.1 | 9.2 ± 0.1 | 9.2 ± 0.1 | 9.2 ± 0.1 | 9.2 ± 0.1 |
| Initial weight (g) | 4.4 ± 0.1 | 4.4 ± 0.1 | 4.4 ± 0.1 | 4.4 ± 0.1 | 4.4 ± 0.1 | 4.4 ± 0.1 |
| Final length (cm) | 13.6 ± 0.9 | 13.6 ± 0.7 | 13.5 ± 0.8 | 14.3 ± 0.7 | 14.2 ± 0.6 | 13.3 ± 0.9 |
| Final weight (g)* | 15.5 ± 3.6a | 16.1 ± 2.8a | 16.7 ± 2.8ab | 17.6 ± 2.9b | 17.2 ± 3.4b | 15.1 ± 3.0a |
| Net length gain (cm) | 4.4 ± 0.6 | 4.4 ± 0.9 | 4.3 ± 0.8 | 5.1 ± 0.5 | 5.0 ± 0.6 | 4.1 ± 0.9 |
| Net weight gain (g) | 11.1 ± 1.6 | 11.7 ± 1.2 | 12.3 ± 0.9 | 13.2 ± 1.3 | 12.8 ± 31.7 | 10.7 ± 1.0 |
| SGR (%) | 0.83 ± 0.3 | 0.85 ± 0.3 | 0.85 ± 0.4 | 0.89 ± 0.3 | 0.88 ± 0.4 | 0.82 ± 0.3 |
| Survival (%)* | 78.3 ± 2.6d | 63.3 ± 3.1c | 48.3 ± 2.9a | 60 ± 2.0c | 58.5 ± 2.8b | 58.3 ± 4.0b |
| Biomass (g) | 730.3 ± 12.1 | 610.6 ± 9.8 | 471.4 ± 10.3 | 632.0 ± 13.2 | 600.4 ± 11.1 | 530.1 ± 9.4 |
M-Mean; SD- Standard Deviation
*p < 0.05; a, b, c, d. Means with the same superscript do not differ from each other (Duncan’s test)
Figure 1: Growth performance of F. indicus fed D. salina diets
Water Quality Parameters
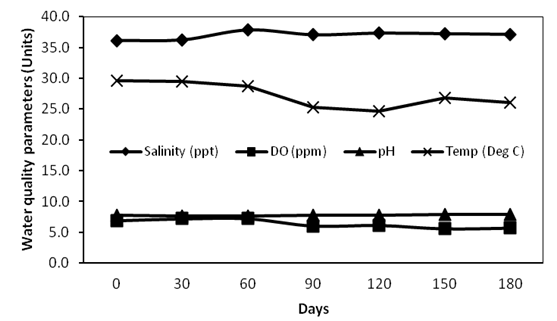
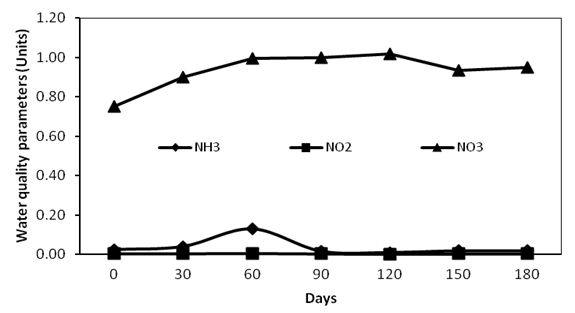
Data on physical and chemical water quality parameters observed during the culture period is depicted in Figure 2, 3. These parameters were found to be within the range suitable for shrimp growth and there was no significant difference (p > 0.05) on parameters between control and treatment tanks.
Figure 2: Physical water quality parameters during the culture period
Figure 3: Chemical water quality parameters during the culture period
Feed Utilization
Details of feed utilization study are presented in Table 2. Protein Efficiency ratio and apparent protein digestibility were found to be high in T3 when compared to control and other treatments. Feed conversion ratio was low in T3. Significant difference (p < 0.05) in feed conversion efficiency was observed between control and T3 but there was no significant difference in feed consumption, excretion, assimilation and protein digestibility between control and treatments.
Table 2: Feed utilization of shrimp fed D. salina diets
| Parameters | Control M ±SD | T1 M ± SD | T3 M ± SD | T3 M ± SD | T4 M ± SD | T5 M ± SD |
|---|---|---|---|---|---|---|
| Feed consumption (g)NS | 1 ± 0.0 | 1 ± 0.0 | 1 ± 0.0 | 1 ± 0.0 | 1 ± 0.0 | 1 ± 0.0 |
| Excretion (g)NS | 0.1 ± 0.1 | 0.1 ± 0.0 | 0.1 ± 0.1 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 |
| Assimilation (g)NS | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.7 ± 0.1 |
| FCE (%)* | 2.90 ± 0.3c | 2.40 ± 0.4b | 2.20 ± 0.3b | 1.80 ± 0.5a | 2.20 ± 0.3b | 2.10 ± 0.4a |
| Protein efficiency ratioNS | 2.57 ± 0.7 | 2.28 ± 0.6 | 2.28 ± 0.6 | 3.14 ± 0.7 | 2.85 ± 0.6 | 2.00 ± 0.4 |
| Protein digestibility (%)NS | 78.9 ± 1.8 | 79.4 ± 1.5 | 80.0 ± 2.2 | 82.0 ± 2.6 | 81.1 ± 1.8 | 78.9 ± 1.7 |
*p < 0.05; NS- p > 0.05; M- Mean; DS- Standard Deviation; FCE-Feed conversion efficiency
a, b, c. Means with the same superscript do not differ from each other (Duncan’s test)
Physical Quality of Shrimp
Details on physical quality of shrimp after culture are presented in Table 3. Soft shell and loose shell percentage was low in D. salina fed shrimps compared to control and the observed difference between control and treatments was significant (p < 0.01). Shrimp colour and taste were found to be increased in higher dose of D. salina fed shrimps.
Table 3: Physical quality of shrimp fed D. salina diets
| Parameters | Control M ± SD | T1 M ± SD | T2 M ± SD | T3 M ± SD | T4 M ± SD | T5 M ± SD |
|---|---|---|---|---|---|---|
| Soft Shell (%)* | 4.2 ± 0.6 | 3.0 ± 0.3 | 6.9 ± 2.1 | 5.6 ± 1.1 | 2.9 ± 0.9 | 5.0 ± 2.1 |
| Loose Shell (%)* | 27.7 ± 2.3c | 20.7 ± 3.1a | 24.1 ± 1.9b | 22.2 ± 2.5b | 20.0 ± 2.8a | 20.0 ± 1.8a |
| Hard Shell (%)* | 68.1 ± 2.5a | 76.3 ± 3.4b | 69.0 ± 3.6a | 72.2 ± 2.6b | 77.1 ± 1.6b | 75.0 ± 2.9b |
| Shrimp colour (cooked) | light | light | light | Dark | Dark | Dark |
| Taste | Bland | Bland | Light sweet | Light sweet | Light sweet | Light sweet |
*p < 0.05; M- Mean; DS- Standard Deviation
a, b, c. Means with the same superscript do not differ from each other (Duncan’s test)
β -Carotene Analysis
Data on β-carotene analysis is shown in Table 4. The average β-carotene level in D. salina is found to be 0.5%. The residual β-carotene content in shrimp carcass after experiment is found to be negligible.
Table 4: β-carotene content in D. salina, feeds and carcass
| Parameters | Control | T1 | T2 | T3 | T4 | T5 |
|---|---|---|---|---|---|---|
| β-carotene in D.salina ( % ) | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| β-carotene in Feed ( % ) | 0 | 0.25 | 0.5 | 1.0 | 2.0 | 7.0 |
| β-carotene in carcass ( % ) | 0 | 0.000057 | 0.000063 | 0.00003 | 0.000044 | 0.000046 |
Proximate Composition Analysis
Results on proximate composition analysis of shrimp carcass are shown in Table 5. Dry matter, moisture, protein, lipid, ash and nitrogen free extract (NFE) contents did not vary significantly (P > 0.01) between control and treatments.
Table 5: Proximate composition of F. indicus fed D. salina diets
| Parameters | Control M ± SD | T1 M ± SD | T2 M ± SD | T3 M ± SD | T4 M ± SD | T5 M ± SD |
|---|---|---|---|---|---|---|
| Dry matter (%)NS | 90.1 ± 00 | 89.9 ± 0.1 | 89.7 ± 0.3 | 90.5 ± 0.2 | 89.9 ± 0.1 | 89.9 ± 0.4 |
| Moisture (%)NS | 9.9 ± 00 | 10.2 ± 0.1 | 10.3 ± 0.3 | 9.6 ± 0.2 | 10.2 ± 0.1 | 10.1 ± 0.4 |
| Protein (%)NS | 71.2 ± 0.4 | 69.2 ± 0.5 | 69.4 ± 0.6 | 68.7 ± 1.0 | 69.6 ± 0.3 | 69.8 ± 1.3 |
| Lipid (%)NS | 4.9 ± 0.6 | 5.9 ± 00 | 4.6 ± 00 | 5.1 ± 0.3 | 4.8 ± 0.1 | 4.9 ± 0.1 |
| Ash (%)NS | 11.5 ± 00 | 11.7 ± 0.1 | 12.1 ± 00 | 11.6 ± 0.1 | 11.8 ± 0.4 | 12 ± 0.4 |
| NFE (%) | 2.6 ± 0.9 | 3.1 ± 0.5 | 3.6 ± 0.3 | 4.1 ± 1.6 | 3.7 ± 0.4 | 3.1 ± 0.3 |
NS p > 0.01; M-Mean: SD- Standard Deviation
Discussion
Results of the present study show that dietary incorporation of D. salina brought significant effect on growth performance in white shrimp, F. indicus and a dose at 2% inclusion in diet exhibited high growth rate compared to control. Similar growth enhancement was also reported in tiger shrimp, Penaeus monodon when D. salina extract was added into the supplementary feed[3,4]. Supplementation of D. salina was not only improved growth, but also enhanced health, immunity and disease resistant in tiger shrimp. Growth promoting effect of D. salina was also reported in pacific white shrimp, Litopenaeus vannamei when carotenoid compounds were supplemented[2,5]. Therefore, it is suggested that the enhanced growth observed in F. indicus is due to incorporation of D. salina in the feed. Studies show that the dose of D. salina required for growth enhancement in tiger prawn and pacific white shrimp are varied[3,4]. These finding clearly reveal that the growth promoting effect of D. salina is dose dependent and species specific.
Several reports suggest that crustaceans have the metabolic activity to introduce structural modifications into carotenoids that they obtain from their diet, in particular, by introducing hydroxy groups at C(3) and C(3’) and keto groups at C(4) and C(4’). Thus, canthaxanthin (β, β-carotene- 4,4’-dione), zeaxanthin (β, β-carotene- 3,3’-diol) and even β-carotene can undergo metabolic conversion into astaxanthin[16,17]. Therefore, it can be suggested that growth promoting effect of D. salina found on F. indicus may be due to the presence of β-carotene content in it.
Water quality parameters recorded during the study was found to be within the suitable range required for the growth of white shrimp[18]. The declining trend of water temperature during culture period was due to the start of winter season. Low concentrations of nitrate observed during culture period suggest the oxidation of ammonia[19]. According to Avnimelech, et al[20], only about 25% of the feed nutrients are converted into harvestable products hence contributing to high nitrogen residues in tank water, especially total ammonia nitrogen (TAN), which is the sum of both ammonia and ammonium which will adversely affect shrimp growth. This may be a reason for the elevated ammonia level recorded during later phase of culture in present study.
Result of the feed utilization study shows that incorporation of D. salina in diets did not affect feed consumption and assimilation. However, protein digestibility was found to be increased slightly in fast growing shrimp and this may be due to the better digestion of food. Proximate composition analysis of meat shows that there is no significant difference in shrimp meat quality which indicates that incorporation of D. salina did not influence meat quality of white prawn. Similar studies have also been reported in shrimp when feed additives are supplemented through diets[21,22].
In shrimp culture industries, color is one of the major factors, which determines the market price of black tiger shrimp (Penaeus monodon) in the international market[23]. Supplementation of carotenoid pigments into diets has been demonstrated to yield shrimp colour and higher pigments in shrimp[15-17]. Moreover, carotenoid pigments have positive effect on the immunological and stress response[24]. Several studies have been made to find alternative sources of astaxanthin and other carotenoids such as yeast, Phaffia sp. and many species of algae[25,26]. In the present study also D. salina diet fed shrimp showed better color than that of the control. Therefore, it is strongly opined that >D. salina has the ability to improve shrimp colour in white shrimp, F. indicus.
It is considered that penaeid shrimp cannot biosynthesize carotenoids from mevalonic acid, but can alter dietary carotenoids by oxidation and deposit them in their tissues Tanaka, et al[27] reported on the metabolism of carotenoids in kuruma shrimp and suggested that some of the dietary carotenoid pigments such as astaxanthin, β-carotene, isocryptoxanthin, echinenone, canthaxanthin, phoenicoxanthin, zeaxanthin and 4-ketozeaxanthin were converted into astaxanthin in shrimp body. Especially, astaxanthin was the most effective substance for pigmentation in shrimp when compared with β-carotene and canthaxanthin[28]. However, Chien et al[17] studied the pigmentation of the black tiger shrimp by feeding the shrimp with diets containing different carotenoid sources, e.g., β-carotene, Spirulina, Phaffia yeast and krill oil. A marked increase of carotenoid content in the carapace was observed in the group fed with Spirulina supplemented diets and suggested that zeaxanthin, which is the major carotenoid in Spirulina, was rapidly converted to astaxanthin. Moreover, 125 mg/kg of synthetic β-carotene or 125–175 mg/kg β-carotene extracted from D. salina have been reported as a pigment sources in black tiger shrimp and demonstrated that black tiger shrimp has the metabolic ability to converted β-carotene into astaxanthin[3]. Based on above observations, it is suggested that F. indicus has the ability to convert the carotenoid content in D. salina and this may be a reason for the better colour development in F. indicus.
From previous reports, dietary carotenoids were converted into astaxanthin and deposited in the shrimp body in free form by the association with protein and exists as carotenoprotein and esterifed forms which are predominantly a mono ester and di ester of long-chain fatty acids[29]. However, the incorporation of astaxanthin esters in test diets seemed less effective than the free form as reported by[17]. The results of the present study shows that D. salina incorporated diet fed shrimp had better colour than that of the control shrimp. Hence it is suggested that the carotene content in D. salina plays an important role on pigmentation in white shrimp as in the case of other shrimps.
Result of the present study show that shrimp body composition was not influenced by D. salina incorporation. The light sweet taste of treatment shrimp can be correlated to the high saline conditions of the Red Sea water used for culture. HPLC analysis on residual β-Carotene content in meat of experimental shrimp shows that presence of β-carotene content at very low level and it did not show significant difference from that of the control. The enhanced color noticed in the shrimp fed D. salina did not change during cooking and it is suggested that the developed color will not change while cooking. In conclusion, it is suggested that carotene content in D. salina play a significant role in enhancing growth and coloration in white shrimp, F. indicus.
Methods
The study was conducted for a period of 180 days in fiber glass tanks (500L) at the University Fish Farm at Obhur, Jeddah. There were control and treatment for the study and both were laid out in a completely randomized design. Healthy and uniform size juvenile (4.4 ± 0.1; 9.2 ± 0.1 cm) produced at the Farm Hatchery were stocked at the rate of 20 pieces/tank. Dry powder of D. salina was procured from National Aquaculture Group (Naqua), Jeddah, Saudi Arabia and incorporated at 0.5, 1.0, 2.0, 4.0 and 8.0% in the diet and designated as treatments T1, T2, T3, T4 and T5 respectively. D. salina free diet was considered as control. A standard fishmeal based pellet feed (Naqua, Jeddah) having 35% protein in diet was selected for D. salina incorporation and the details of feed ingredients are shown in Table 6. Required quantity of D. salina (powder) was thoroughly mixed with soaked feed and extruded out through a pelletizer having 2m in the die. The pellets were then oven dried at 60°C and kept it in airtight containers for storage. Feeding was done at 5% of the biomass daily at 6:00 AM, 1 PM and 6 PM. Water exchange was done at the rate of 20% every day. Shrimp were sampled fortnightly to record their total length and weight and the feed quantity was re adjusted after every sampling. Specific growth rate was calculated as Loge W2-LogeW1/T2-T1 (where W2 is the weight of shrimp at time T2 and W1 is the weight of shrimp at time T1).Water quality parameters such as temperature, dissolved oxygen, pH and salinity (HAAC, HQ40d multiply portable device meter) were recorded daily. Ammonia (unionized), nitrates (NO3) and nitrites (NO2) were recorded (HAAC, device colorophotometer – DR 3900) weekly. On 180th day, the experiment was terminated and total biomass and survival was calculated.
Table 6: Feed ingredients in the supplementary feed
| Ingredients | Incorporation (%) |
|---|---|
| Fish meal | 18.00 |
| Soya meal | 40.00 |
| Wheat | 31.78 |
| Fish oil | 3.50 |
| Sea weed powder | 0.50 |
| Mono sodium phosphate | 0.38 |
| Mono calcium phosphate | 0.50 |
| Yeast autolyzate | 0.50 |
| Soya lecithin oil | 2.53 |
| Anti-oxidant | 0.10 |
| Anti-mould | 0.15 |
| Binder | 1.00 |
| Vitamin premix | 0.35 |
| Mineral premix | 0.25 |
| DL Methionine | 0.27 |
| Vitamin C | 0.14 |
| Lysine (Amino acid) | 0.05 |
| Total (g) | 100 |
A short term laboratory experiment was conducted in fiber glass tanks (50 liter) using 10 shrimps (10.0 ± 1.2 g) for 30 days to assess the feed intake, conversion efficiency, and apparent protein digestibility of the control and treatment shrimp. For this, shrimp were collected from the experimental tanks and provided with weighed amount of feed for 2 hours daily between 7.00 am and 7:00 pm. The unconsumed feed and fecal matter were siphoned out separately after 1hour of feeding. The pooled, dried and weighed faecal matter was used for nutrient analysis. Feed conversion efficiency and nutrient digestibility were calculated as: Feed conversion efficiency (FCE %) = Wet weight gain (g) / Feed consumed (g) x 100; Apparent protein digestibility (%) = Protein in feed - Protein in excreta / Protein in feed x100.
A panel of experts evaluated the physical quality of shrimp such as colour, loose shell, soft shell, hard shell and taste after the experiment. Proximate analysis of shrimp carcass was analyzed for the estimation of protein, lipid, glycogen, fiber and ash[30]. Experimental feed, shrimp samples and dry powder of D. salina were subjected for the estimation of β-carotene content by High Pressure Liquid Chromatography (HPLC)[31] and the samples were analyzed at IDAC lab, Riyadh, Saudi Arabia. One way analysis of variance (ANOVA) was employed to find out the statistical difference in growth, water quality parameters and physical quality of shrimp between control and treatment[32].
Acknowledgment: The author, Osamah A Ahmad is gratefully thankful for the research grant awarded by KACST (AT-34-232) for post graduate studies. Authors are thankful to the Dean Faculty of Marine Sciences and the Managing Director, Naqua for the facilities provided.
References
- 1. Wang, X., Wille, N.R., Wadstrom, T. Astaxanthin-rich algal meal and vitamin C inhibit Helicobacter pylori infection in Balb/cA mice. (2000) Antimicrob Agents Chemother 44(9): 2452- 2457.
- 2. Arredondo-Figueroa, J.L., Pedroza-Islas, R., Ponce-Palafox, J.T., et al. Pigmentation of Pacific white shrimp (Litopenaeus vannamei, Boone, 1931) with esterified and saponified carotenoids from red chili (Capsicum annuum) in comparison to astaxanthin. (2003) Rev Mex Ing Quim vol 2(2): 101- 108.
- 3. Boonyaratpalin, M., Thongrod, S., Supamattaya, K., et al. Effect of β-carotene source, Dunaliella salina, and astaxanthin on pigmentation, growth, survival and health of Penaeus monodon. (2001) Aquacult Res 31(s1): 182- 190.
- 4. Supamattaya, K., Kiriratnikoma, S., Boonyaratpalin, M., et al. Effect of a Dunaliella extract on growth performance, health condition, immune response and disease resistance in black tiger shrimp (Penaeus monodon). (2005) Aquaculture 248(1-4): 207- 216.
- 5. Linan-Cabello, M.A., Paniagua-Michel, J., Zenteno-Savin, T. Carotenoids and retinal levels in captive and wild shrimp, Litopenaeus vannamei. (2003) Aquacult Nutr 9(6): 383- 389.
- 6. Pan, C.H., Chien, Y.H., Hunter, B. The resistance to ammonia stress ofPenaeus monodon Fabricius juvenile fed diets supplemented with astaxanthin. (2003) J Exp Mar Biol Ecol 297(1): 107- 118.
- 7. Mcnulty, H., Jacob, R.F., Mason, R.P. Biologic activity of carotenoids related to distinct membrane physicochemical interactions. (2008) Am J Cardiol 101(10A): 20D- 29D.
- 8. Britton, G. Structure and properties of carotenoids in relation to function. (1995) FASEB J 9(15): 1551- 1558.
- 9. Nielsen, B.R., Mortensen, A., Jorgensen, K., et al. Singlet versus triplet reactivity in photo degradation of c40 carotenoids. (1996) J Agric Food Chem 44(8): 2106- 2113.
- 10. Palozza, P., Barone, E., Mancuso, C., et al. The protective role of carotenoids against 7-keto-cholesterol formation in solution. (2008) Mol Cell Biochem 309(1-2): 61- 68.
- 11. Oren, A. A hundred years of Dunaliella research, 1905-2005. (2005) Saline Syst 1: 2- 15.
- 12. Tafreshi, A.H., Shariati, M. Dunaliella biotechnology: methods and applications. (2009) J Appl Microbiol 107(1): 14- 35.
- 13. Ben-Amotz, A., Polle, J.E.W., Subba Rao, D.V. The algae Dunaliella: Biodiversity, Physiology, Genomics and Biotechnology. (2009) New Hampshire: Science Publishers.
- 14. Stottrup, J.G., McEvoy, L.A. Live Feeds in Marine Aquaculture. (2003) Oxford: Blackwell Publishing Ltd.
- 15. Dufosse, L., Galaup, P., Yaron, A., et al. Microorganisms and microalgae as sources of pigments for food use: a scientific oddity or an industrial reality. (2005) Trends Food Sci Technol 16(9): 389- 406.
- 16. Liao, W.L., Nur-E-Borhan, S.A., Okada, S., et al. Pigmentation of cultured black tiger prawn by feeding with a Spirulina supplemented diet. (1993) Nippon Suisan Gakk 59(1): 165- 169.
- 17. Chien, Y.H., Jeng, S.C. Pigmentation of kuruma prawn, Penaeus japonicus Bate, by various pigment sources and levels and feeding regimes. (1992) Aquaculture 102(4): 333- 346.
- 18. Wickins, J.F. The tolerance of warm water prawn to recirculated water. (1976) Aquaculture 9(1): 19- 37.
- 19. Cohen, J.M., Samocha, T.M., Fox, J.M., et al. Characterization of water quality factors during intensive raceway production of juvenile Litopenaeus vannamei using limited discharge and biosecure management tools. (2005) Aquacult Eng 32(3-4): 425- 442.
- 20. Avnimelech, Y., Ritvo, G. Shrimp and fish pond soils, processes and management. (2003) Aquaculture 220(1-4): 549- 567.
- 21. Nakagawa, H., Gomez-Diaz, G. Usefulness of Spirulina sp. meal as feed additive for giant freshwater prawn, Macrobrachium rosenbergii. (1995) Aquacult Sci 43(4): 521- 526.
- 22. Jaime-Ceballos, B., Villareal, H., Garcia, T., et al. Effect of Spirulina platensis meal as feed additive on growth, survival and development in Litopenaeus schmitti shrimp larvae. (2005) Rev Investig Mar 26(3): 235- 241.
- 23. Latscha, T. Carotenoids in aquatic animal nutrition. (1991) Proceeding of the Aquaculture feed processing and Nutrition Workshop, Bankok, Thailand 19-25: 68- 78
- 24. Chien, Y., Pan, C., Hunter, B. The resistance to physical stresses by Penaeus monodon juveniles fed diets supplemented with astaxanthin. (2003) Aquaculture 216(1-4): 177- 191.
- 25. Sanderson, G.W., Jolly, S.O. The value of Phaffia yeast as a feed ingredient for salmonid fish. (1994) Aquaculture 124(1-4): 193-200.
- 26. Boonyaratpalin, M., Supamattaya, K., Borisuth, C. The immune system in black tiger shrimp, Penaeus monodon Fabricius: VIII. Effect of astaxanthin on blood parameters, immune system and disease resistance in black tiger shrimp (Penaeus monodon Fabricius). (2000) Songklanakarin J Sci Technol 22: 633- 639.
- 27. Tanaka, Y., Matsuguchi, H., Katayama, T., et al. The biosynthesis of astaxanthin-XVIII. The metabolism of carotenoids in the prawn, Penaeus japonicus Bate. (1976) B Jpn Soc Sci Fish 42(2): 197-202.
- 28. Estermann, R. Biological functions of carotenoids. (1994) Aquaculture 124(1-4): 219-222.
- 29. Yamada, S., Tanaka, Y., Sameshima, M., et al. Pigmentation of prawn (Penaeus japonicus) with carotenoids: I. Effect of dietary astaxanthin, β-carotene and canthaxanthin on pigmentation. (1990) Aquaculture 87(3-4): 323-330.
- 30. AOAC. Official method of analysis of the Association of official analytical chemists. Horwitz, W., Ed. 17th edition. (2000) Washington, DC.
- 31. Van Vliet, T., Van Schaik, F., Van Schoonhoven, J., et al. Determination of several retinoids, carotenoids and E- vitamins by high-performance liquid chromatography. Application to plasma and tissues of rats fed a diet rich in either beta-carotene or canthaxanthin. (1991) J Chromatogr 553(1-2): 179-186.
- 32. Snedecor, G.W., Cochran, W.G. Statistical methods. (1967) Oxford and IBH Publishing Company, Calcutta, 593.